Nicotinamide Riboside vs NMN: Debunking the NMN Science
Niagen® (nicotinamide riboside) and nicotinamide mononucleotide (NMN) are two well-known NAD+ precursors, but NMN falls short as a safe and effective supplement to elevate NAD+ (nicotinamide adenine dinucleotide).
In recent years, NAD+ continues to be at the forefront of research in aging, health, and disease.
However, the popularity of NAD+ research also opened the door for misinformation about NAD+ and NAD+ precursors.
For example, an NAD+ precursor is a “building block” for the cell to build NAD+. However, NMN is technically a precursor to nicotinamide riboside, which, in turn, becomes NAD+. Given that nicotinamide riboside (NR for short) is more direct, this distinction is important to make when it comes to efficiency.
Also, nicotinamide riboside carries a long list of global regulatory bodies that accept or approve its use.
In contrast, NMN has not been reviewed for safety by the FDA in the U.S. or by any other international regulatory body. Yet, NMN is commercially available.
So, which one should you trust? The evidence overwhelmingly favors Niagen®, but here’s a breakdown to adequately assess these NAD+ precursors in detail.
Researchers prefer Niagen® (nicotinamide riboside) over NMN, nearly 3 to 1.
According to Clinicaltrials.gov, a government-sponsored database of human clinical studies, as well as the World Health Organization (WHO) International Clinical Trials Registry, there are a total of 48 ongoing studies involving Niagen® nicotinamide riboside vs. a mere 17 for NMN. That’s a near 3:1 ratio.
These numbers show that the world’s leading academic and medical researchers, investigating the various health benefits of raising NAD+ levels in humans, place their trust and confidence in Niagen® over NMN.
Niagen® (nicotinamide riboside) has twelve published human clinical studies. NMN has two.
Niagen® has been the subject of twelve human clinical studies, providing a broader and far more convincing foundation of scientific evidence as the preferred NAD+ booster.
In contrast, NMN has been the subject of only two published clinical studies.
NMN’s First Human Study:
The first study, published in the Endocrine Journal in 2020, failed to assess the effect of NMN supplementation on NAD+ levels adequately.
Researchers conducted a small, non-blinded, uncontrolled (no placebo) trial consisting of 10 healthy Japanese male subjects aged 40-60. The study administered NMN over the course of three visits, each spaced more than a week apart. Subjects received a single 100, 250, or 500mg dose of NMN.
The study concluded that NMN supplementation shows no serious adverse effects and tolerability in moderately high doses. Still, it did not assess the effect on NAD+ levels or the safety of daily NMN supplementation over any significant period of time.
NMN’s Second Human Study:
The second study, published in the journal Science showed better results. NMN supplementation significantly increased NAD+ levels and increased muscle insulin sensitivity and signaling in prediabetic women.
However, a major limitation of this study lies in the researchers’ failure to randomize the participants properly.
At baseline, the placebo group and NMN group were vastly different with respect to metabolic health. The average level of liver fat in the placebo group was over twice that in the NMN group.
Ensuring quality randomization of participants in experimental design prevents accidental bias. In the case of NMN’s second human study, improper randomization likely resulted in an overpromising improvement in the NMN group.
Niagen® (nicotinamide riboside) has nine published human clinical studies demonstrating that it effectively increases NAD+ levels. NMN has one.
Overall, to date, a collection of human clinical studies comprehensively shows that Niagen® effectively and safely increases NAD+ levels. The continued use of Niagen® in these trials further reflect the trust that the precursor holds in the scientific community. Overwhelmingly, the body of research favors Niagen® as the best choice for a NAD+ precursor.
First Human Niagen® Study That Demonstrates Effectiveness:
A study published in Nature Communications in 2016 reports that single oral doses of 100mg, 300mg, and 1000mg of Niagen® can safely elevate NAD+ levels. Researchers investigated the effects of NR supplementation in 12 healthy men and women, establishing both its safety and efficacy as an NAD+ precursor in humans.
Second Human Niagen® Study That Demonstrates Effectiveness:
A study published in PLOS One in 2017 monitored Niagen® effects with a gradual increase in dosage. The study administered 250mg of Niagen® to eight healthy volunteers on Days 1 and 2, then gradually administered higher doses each subsequent day after. On days 7 and 8, the study administered a peak dose of 1000mg twice daily. The study concluded on Day 9, showing oral administration of Niagen® to be well-tolerated in humans with no adverse effects.
Third Human Niagen® Study That Demonstrates Effectiveness:
A study published in Nature Communications in 2018 shows Niagen® supplementation is well-tolerated and effectively elevates NAD+ in a group of healthy middle-aged and older adults. The subjects were 60 healthy men and women between the ages of 55-79. The study administered a dosage of 500mg twice per day for six weeks in a randomized, placebo-controlled, and crossover designed trial.
Fourth Human Niagen® Study That Demonstrates Effectiveness:
A study published in the American Journal of Clinical Nutrition in 2018 concluded that 2000mg of daily Niagen® supplementation over a period of 12 weeks is safe. In addition, the subjects showed an increase in NAD+ metabolites in urine samples.
The researchers administered 1000mg of Niagen® twice daily over a period of 12 weeks in a randomized, placebo-controlled, double-blind, parallel-group designed trial. The subjects consisted of 40 healthy, obese men ranging in ages of 40-70.
Fifth Human Niagen® Study That Demonstrates Effectiveness:
A clinical trial published in Scientific Reports in 2019 concludes Niagen® both safely and effectively increases NAD levels in a dose-dependent manner in healthy overweight adults. The study used an 8-week randomized, double-blind, placebo-controlled trial to conduct its evaluation. After two weeks, the study results are as follows:
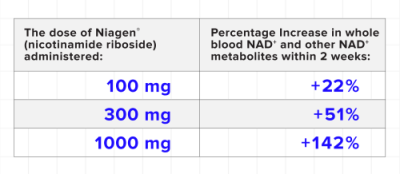
Sixth Human Niagen® Study That Demonstrates Effectiveness:
A study published in Cell Reports in 2019 investigated the effects of Niagen® on skeletal muscle. The study supplemented 12 marginally overweight older men with 1000mg of Niagen® daily for 21 days. The study used a randomized, double-blind, placebo-controlled, crossover designed trial and concluded, “oral nicotinamide riboside increased human skeletal muscle NAAD, a sensitive marker of increased NAD+ metabolism.”
Seventh Human Niagen® Study That Demonstrates Effectiveness:
A study published in The American Journal of Clinical Nutrition in 2020 investigated the effects of Niagen® on metabolic health, muscle metabolism, and mitochondrial function. The study was a randomized, double-blinded, placebo-controlled, crossover trial of 13 healthy overweight and obese adults. Similar to the previous study, NR supplementation significantly increased markers of enhanced NAD+ metabolism in human skeletal muscle.
Eighth Human Niagen® Study That Demonstrates Effectiveness:
A study published in Molecular Systems Biology in 2020 investigated the effect of Niagen® in combination with other supplements on liver fat. The study combined NR with L-serine, N-acetyl-L-cysteine, and L-carnitine for their potential benefits for people with higher liver fat content. Mathematical modeling results showed increased fat metabolism, decreased glucose metabolism, and increased synthesis/turnover of NAD+, carnitine, and glutathione.
Ninth Human Niagen® Study That Demonstrates Effectiveness:
An ex vivo and pilot clinical study published in The Journal of Clinical Investigation investigated mitochondrial dysfunction in peripheral blood mononuclear cells (PBMCs). The study showed NR increased whole blood NAD+ levels and the mitochondrial respiration rate of PBMCs.
Nicotinamide riboside can enter cells directly. NMN cannot.
Nicotinamide riboside, once absorbed into the body, enters cells directly, whereas NMN cannot. A collection of published studies conducted by some of the field’s leading researchers demonstrate that NMN cannot enter cells directly; rather, it must be converted to nicotinamide riboside first.
For example, a study published in Nature Communications in 2016 on NMN’s metabolism in mammalian cells concluded NMN cannot directly enter the cell.
Nicotinamide riboside and NMN are chemically identical, with the exception of one phosphate group present on NMN. The study demonstrates that this additional phosphate group must be removed from NMN, converting it into nicotinamide riboside before it can enter the cell.
Similarly, NAD+ supplementation provides no advantage over nicotinamide riboside because NAD+ is too large and contains multiple phosphate groups. NAD+ must be broken down into individual parts before entering the cell; then, it reforms back into NAD+.
However, a study published in Nature Metabolism in 2019 claimed to have identified a transport protein for NMN in the small intestine of mice. But researchers Mark S. Schmidt & Charles Brenner questioned the validity of this claim, stating there is an absence of evidence for this NMN transporter.
Niagen® nicotinamide riboside has been reviewed and accepted by the leading regulatory authoritative bodies in the world. NMN has not.
United States
-
Niagen® has been successfully reviewed twice under the US Food and Drug Administration’s (FDA) New Dietary Ingredient Notification (NDIN) program.
-
Niagen® was successfully notified to the FDA as Generally Recognized As Safe (GRAS).
Canada
-
Niagen® received approval as a Natural Health Product by Health Canada.
Europe
-
Niagen® received approval as a Novel Food Ingredient by the European Commission.
Australia
-
Niagen® received approval as a permissible ingredient in Complementary Medicines by the Therapeutic Goods Administration of Australia.
These approvals and acceptance by the world’s leading authoritative regulatory bodies is a clear, unequivocal recognition of the quality of the science, the safety of the ingredient, and the reproducibility of the production process.
In comparison, no NMN ingredient or product holds these qualifiers.
Niagen® nicotinamide riboside bears the NSF Certified for Sport® seal. NMN does not.
Tru Niagen®, ChromaDex’s consumer product of nicotinamide riboside, bears the NSF Certified for Sport® seal.
The NSF Certified for Sport® includes a certification that Tru Niagen® is made to current Good Manufacturing Practice (cGMP) standards, testing to confirm the level of Niagen® in the product is consistent with the amount claimed on the label and absent of over 270 athletic banned substances and harmful contaminants.
On the other hand, the lack of regulatory review of NMN precludes NMN products from bearing the NSF Certified for Sport® seal.
After reviewing the facts, the choice is clear.
Niagen® nicotinamide riboside provides a sound basis for confidence. The studies behind it are overwhelmingly positive, and the regulatory approvals are ironclad.
In contrast, only one study suggests NMN is a safe and effective method for elevating NAD+ levels in humans. And NMN’s lack of review and acceptance by regulatory bodies is a safety concern.
It all comes down to how much you are willing to gamble on a questionable product.
We covered a lot in this comparison. And perhaps you’re still mulling it over. Below is a brief summary of our comparison to help you get a better bird’s eye view of both these ingredients.



