What Is a Clinical Trial for Supplements?

At one time, drugs and therapies were highly unregulated. All you had was the good word of your doctor. Active ingredients varied from batch to batch, and side effects weren’t discovered until after you received your treatment.
But starting from the 1930s, medical researchers began evaluating the claims behind different kinds of drugs and therapies. Researchers created a system known as a clinical trial to help standardize medicine, herbs, and supplements.
Clinical trials are research studies performed in people to determine whether a test or treatment is safe and effective.
Two types of clinical trials.
1. Observational studies.
Observational studies involve monitoring people over time, investigating how their behaviors or actions possibly lead to some health outcomes.
2. Intervention studies.
Intervention studies involve exposing subjects to an intervention, like the ingestion of a supplement. Uncontrolled studies include only the intervention without a juxtaposed control or placebo group. On the other hand, controlled studies do involve either a control group or a placebo group for comparison.
A randomized, controlled trial (or RCT) is considered the “gold standard” of human research since it involves a control group, and subjects are randomly assigned to either the placebo or the studied intervention. Both researchers and subjects are blinded to the intervention's administration, meaning neither is aware of the group assignments. A blind study minimizes the risk of bias, securing the observed results as objective data.
Supplements vs. drugs.
Typically, clinical trials for drugs advance through four phases of testing, undergoing exorbitant cost. According to Nature Reviews Drug Discovery, the median expense for a single Phase Three drug trial is $19 million.
However, most studies for nutritional supplements don’t go past more than two phases of testing because they do not assess the prevention, treatment, or cure of an ailment.
How to arrange a clinical trial?
Clinical trials require careful planning and review. To begin a clinical trial, you need a principal investigator, the person who oversees the research. The principal investigator is usually a scientist considered an expert in the field pertaining to the trial. They design the experiment and select a research team for the experiment.
Clinical trials also need sponsors who fund the experiment. Sponsors can range from government agencies to companies and non-profit organizations. Sponsors review the research plan that outlines the subjects to be studied in the trial, the measurements to be taken, and the schedule of procedures.
The IRB (Institutional Review Board) also needs to approve of the clinical trial before it begins. An IRB consists of people chosen by the health care center that provides the participants for the trial. Each IRB usually includes five members and must consist of one of the following:
-
one scientist,
-
someone who’s not a scientist, and
-
someone who’s not from the health care center.
Each IRB reviews the available data to assess the safety and potential risks of testing the substance in humans. Most new substances must pass substantial preclinical safety testing in an IRB review before they are approved for use in humans.
Key questions to consider when reviewing the clinical trials for supplements.
Clinical trials can be hard to decipher. Here are some criteria you can review a supplement’s merit behind its advertised clinical trials:
1. Did the clinical study conduct a randomized, controlled trial (RCT)?
Was there a control or placebo group in the trial? Were both the subjects and researchers blinded, meaning were they aware of which subjects received a placebo and which ones received the supplement? These parameters are essential to determine the objectivity of the clinical results.
2. Did the results of the clinical trial show statistical significance?
Statistical significance helps quantify whether a trial result is likely due to chance or due to the real effect of the studied supplement. Usually, the larger the sample size, or power of the study, the less variability you have to contend. The greater the variability with subjects or between subjects to a given intervention, the more difficult it is to show a statistical difference.
3. What was the size or duration of the study?
Speaking of sample size, the best way to maximize your statistical significance is with a longer duration of time and a larger sample size in your test. Checking the number of subjects involved and the length of a study is an excellent way to find if the clinical trial was more or less prone to sampling error. Typically, longer duration studies allow more time for effects to be observed.
4. Was the study published in a peer-reviewed journal?
The peer-review process places a clinical study under the scrutiny of statisticians and scientists with expertise in the subject matter.
While the peer-review process is not perfect, it is a fairly robust and reliable way to ensure that published studies are generally well-designed and not over-interpreted.
Typically, clinical studies without a mention of a publication denote a red flag, being absent of a critiquing process that assesses the merit of an investigation.
5. Did the clinical trial test the branded product sold to consumers?
Perhaps one of the most overlooked details of a clinical study on a supplement is whether the actual marketed product was used. Some supplements tout clinical studies conducted on similar versions of the product and not on the real ingredient or product consumers are using. The best practice is to check if the study cites the product or ingredient by brand or company name.
6. Does the use of the ingredient or product in the clinical trial match the supplement label directions?
Check to see if the dose of the product studied differs from the recommended use on your supplement label. Does it match? What about the administration of the supplement? Does the study show that the supplement was taken orally or intravenously?
Sometimes, researchers test for a range of conditions to better understand the scope of the effects on a supplement. However, the absence of testing the recommended use leaves much to be desired within the clinical data.
7. Does the company provide access to the published study?
Copyright laws prevent companies from providing actual copies of studies to consumers. However, a link to the published journal’s website is fair-game. Many published studies are open-access, at no cost to the consumer.
If a company does not provide any links to their published studies, it might indicate that the company is not familiar with the research, or the study was not done on the same ingredient or product you’re using.
Explore the clinical trials behind Tru Niagen®.
Eight clinical trials fortify the ingredient, Niagen®, all of which have proven the safety and/or efficacy of Niagen®. Ten peer-reviewed manuscripts have been published with more than 30 additional ongoing clinical studies registered on www.clinicaltrials.gov. Below are the studies behind Niagen®.
-
A study published in Nature Communications in 2016 by Trammell et al.
-
A study published in PLOS One in 2017 by Airhart et al.
-
A study published in Nature Communications in 2018 by Martens et al.
-
A study published in the American Journal of Clinical Nutrition in 2018 by Dollerup et al.
-
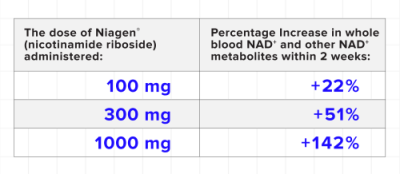
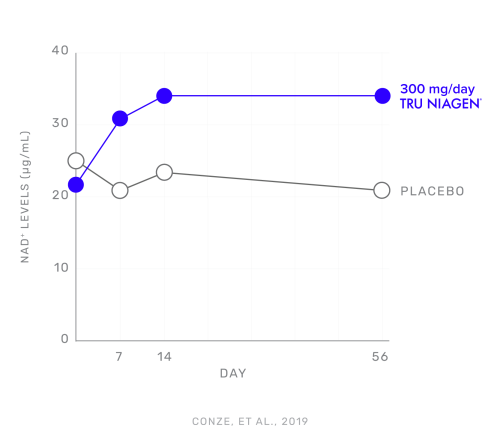
A clinical trial published in Scientific Reports in 2019 by Conze et al.
-
A study published in the Journal of Clinical Endocrinology & Metabolism in 2019 by Dollerup et al.
-
A study published in the Journal of Physiology in 2019 by Dollerup et al.
-
A study published in Cell Reports in 2019 by Elhassan et al.
-
A study published in the American Journal of Nutrition in 2020 by Remie et al.
-
A study published in Molecular Systems Biology in 2020 by Zhang et al.
Many supplements on the market may have been tested in one or two clinical studies, but often these studies do not have the scientific rigor to meet the standards for a peer-reviewed publication.
Tru Niagen® has a global network of independent scientists eager to study its potential benefits in a wide variety of areas.
Thus far, the clinical background shows that there is already a keen interest within the scientific community behind the ingredient, Niagen®, atypical of others. It demonstrates a level of excitement that can only further propel the research of its applicable properties.